Role
PEPPER is a non-profit association (under the French 1901 law) and a public-private platform dedicated to the pre-validation of endocrine disruptors characterization methods. It is supported by the French “Programme of Investments for the Future” (PIA), government departments and industry associations. PEPPER’s mission is to organize and fund scientific research and testing in order to obtain the proofs required by the authorities in charge of international validation (OECD, ECVAM, ISO). Hence the word “pre-validation”.
The idea first emerged in 2014, with the first National Strategy for Endocrine Disruptors in France – a first worldwide. PEPPER was then launched alongside the second French National strategy. Several elements have led to the creation of our organisation, the first of its kind in Europe.
Accelerating research to understand and adapt our risk management and regulation.

Action 41 Introducing a platform to support the validation of methods and tests permitting the identification of endocrine-disrupting effects
Addressing the current confusion and uncertainty
The lists of suspected EDs which need to be assessed are controversial and long – from a couple of dozens to thousand entries. This situation of uncertainty has a damaging impact on public health and environment safety, innovation, products safety and trust in the authorities.

Addressing the lack of test methods
The European Commission has stressed the need for test methods allowing to characterize a substance as an endocrine disruptor. Many mechanisms are indeed at play, depending on age groups, for the human as well as for animal species. Despite some research progress, the range of available methods is incomplete.
A missing link to fast-forward the validation process
Only a small number of methods developed by scientists can effectively be used to regulate, classify or even simply build a consensus. Despite the research progress, the acknowledgement by official authorities is a long and difficult process, as the tests to establish the reliability, repeatability and reproducibility of methods are expensive, non-lucrative for the developers and do not bring any added value to existing research. The fact is that research funding for regulatory purposes is very little developed.
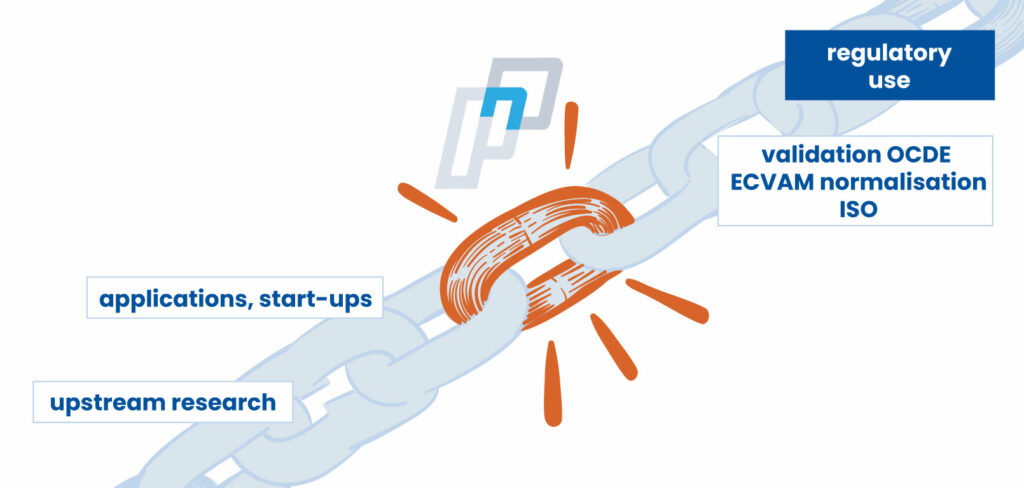
PEPPER’s mission is indeed to carry out these operations, thereby meeting the demand from the EU to facilitate the work of international authorities like the OECD.
The need to mutualise resources
Until recently, there was no funding available to support the validation process. Meanwhile a multitude of stakeholders are confronted to issues pertaining to EDs: health or biodiversity agencies as well as companies manufacturing or putting substances on the market. EU countries should mutualise their resources, as every industry is potentially concerned by endocrine disruption issues. They share the need for mutually acknowledged tools which are validated by international authorities. Mutualisation of testing facilities is also part of the process launched by PEPPER.
Ambitions
To fast-forward translational research
PEPPER serves as a catalyst for translational research, by creating opportunities for operational applications.
To support the test laboratories and biotechnologies in France
Involving laboratories in the testing of methods aims at supporting an industry which is critical to the French innovation ecosystem.
To support evaluation by fostering a stakeholders dialogue
Tools and solutions should meet an existing need and be considered with a practical approach. Between protecting the environment, biodiversity and human health, between scientific progress, existing issues and suspected risks, choices and priorities must be discussed; PEPPER brings together a wide range of stakeholders: authorities, scientists, laboratories, manufacturers, environmental, consumer and health organisations from different countries.
To protect innovation
PEPPER’s work will protect innovation by allowing developers to work according to clear rules on health and environmental safety.
To create a European dynamic
France has been and continue to be instrumental in the advancement of research and discussions on EDs in Europe. PEPPER is one element of this ambition. With organisations from other European countries as well as European and international organisations sitting in its governing bodies, with candidate methods as well as laboratories conducting the tests involving other European countries, PEPPER truly has a European ambition.
First platform of its kind, PEPPER is destined to become a European organisation contributing to fast-forward the validation process.

To bring regulatory science and scientific research closer together
Regulatory science is the sciencewhich is used to base public authorities’ decisions on scientific evidence. A 2019 report from the French Parliamentary Office for evaluation of scientific and technological options (OPECST) on the assessment of risk for human health and the environnement by government agencies recommends to encourage a regular update of existing guidelines to prevent from unnecessary delays in the adoption of new performant and reliable methods and tests. PEPPER’s work will allow for a better dialogue and alignment.
Values
PEPPER was established on the principle that it is possible to work in a collaborative manner around the development of methods for the characterization of EDs.
To accept a diversity of perspectives
To this end, PEPPER will encourage cooperation between stakeholders which may have different agendas and constraints. The association members and the members of its governing bodies must pay attention to each other’s (sometimes diverging) opinions and agree to build a dialogue.
To comply with the principles of scientific integrity
PEPPER works within the ethics rules defined by the European Federation of Academies of Sciences and Humanities ALLEA, namely reliability, honesty, respect, accountability and the vigilance to prevent the “fabrication” of results, falsification and plagiarism.
To stay within its role
PEPPER is committed to deliver tools which will be available to all, leading to common and established foundation for substances assessment and risk management. It is not PEPPER’s role to comment on decisions from the organisations in charge of methods’ validation. Neither is it to assess any substance nor to comment on risk management options. Should a member have to do any of the above, they cannot, in these circumstances, refer to their PEPPER membership.
To work in a « one health » mindset
PEPPER’s work on endocrine disruptors do not address human health separately from the environment and ecosystems. Test methods address one and the other and, preferably, both together.
To work in an « open science » mindset
PEPPER supports “open science” principles as per the OECD definition ; our work will be made publicly available within the limits of intellectual property matters.
