Validation and prevalidation
The test methods are subject to formal validation by the OECD and / or by ECVAM (for in vitro tests) when it comes to substances, or CEN / ISO standardization for media and medical devices.
This validation ultimately gives a guarantee of quality, enforceability and multilateral recognition by member countries, for example of the OECD.
The principle is that all test laboratories following quality procedures, in particular “good laboratory practice” (GLP) will find the same results.
The guarantee of quality goes through tests demonstrating repeatability and reproducibility and requires circular tests. These long and costly operations can be done in a pre-validation structure.
Several definitions of pre-validation exist. For PEPPER, it is a question of ensuring that the methods meet quality criteria, and of constructing the evidence required by the bodies which carry out an internationally recognized validation (OECD, ECVAM, ISO).
Interview n1, Philippe Hubert, Director
Interview, Philippe Prudhon, President
Interview n2, Philippe Hubert, Director
Interview, Ella Atlas, Scientific Council
Operations carried out and financed by PEPPER
Three operational functions are performed and funded by the platform staff and are at the heart of its business :
- the identification and documentation of mature, scientifically sound test methods that meet a need in terms of identifying EDs;
- the organization of tests on these methods, using test laboratories, to assess the repeatability and reproducibility of the results on the basis of “positive” and “negative” substances and by performing ring trials;
- support for method bearers in submitting their methods and the required scientific reports to international validation bodies.
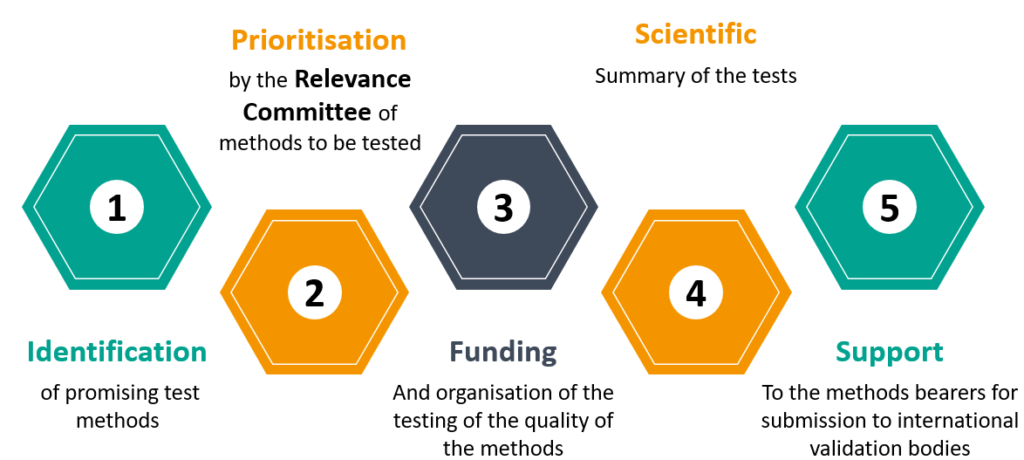
Operational functions involve two decision-making steps that involve governance bodies :
- The decision on whether to follow a pre-validation process to a method, and therefore to organize and finance tests on this method, is a matter for the Relevance Committee.
- The conclusions on the success or failure of the pre-validation of a method are established by the Scientific Council.
The test methods that PEPPER is working on
The test methods targeted by the platform are bioassays. A bioassay consists in exposing a living organism (we speak of “in vivo”) or a cell, or a cellular system (we speak of “in vitro”) to a substance (or an extract from an environment) whose toxicity and mode of action is to be assessed.
PEPPER works on 3 types of tests:
- On infra-cellular or simple cellular systems, where we will for example look to see if a chemical binds to a receptor in the endocrine system. Binding to the estrogen receptor is one of the first uses of these tests for endocrine disruptors. The stakes are often on other receptors;
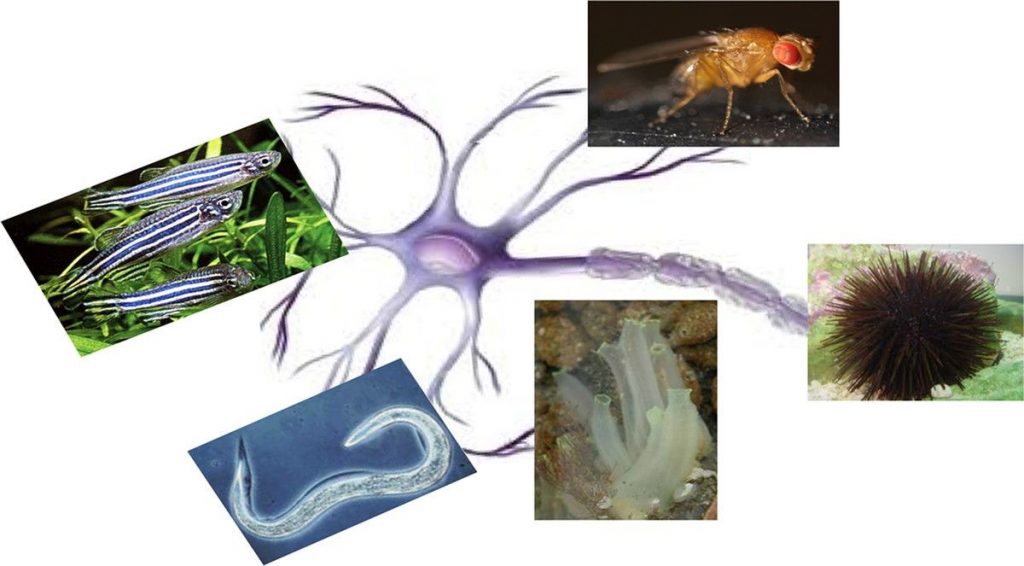
- On more complex cellular systems, such as 2D or 3D tissues, or when micro-technologies and cell culture are used to recreate complex neural architectures in vitro;
- On simple animal models. The zebrafish and its embryo are examples. Other examples include invertebrates which are not regulated by the Directive on the protection of animals used for scientific purpose (Dir 2010/63/EU).


Additional services
The platform offers “additional services” on a billed service basis.
- Organize experiments that will allow the proof of concept of methods that are too immature ;
- Spread scientific watch on emerging methods ;
- Accompanying files after they are submitted to international bodies ;
- Conduct training on pre-validated methods.
PEPPER’s know-how
PEPPER teams knowledge on :
- How to run a bioassay and know the constraints to ensure quality of results ;
- What are the prerequisites and expectations of international organisations ;
- Where are the current issues and the gaps to be filled to characterise endocrine disruptors ;
- Which laboratories can carry out the tests ;
- Where to search for potential bioassays and how to characterize the maturity of a proposed assay.
This is only possible because PEPPER’s teams rely on a wide network of partners, especially those who are part of the Relevance Committee and the Scientific Council.
