Endocrine disruptors are an important and multifaceted societal issue, with scientific, regulatory, economic, risk management and innovation management debates; debates which feature multiple and divergent opinions and analysis.
A long path from scientific to regulatory definition
The words « endocrine disruptor » (ED) were first mentioned by zoologist Theo Colborn on 28th July 1991 in a scientific gathering in Wingspread (USA). The notion derives from observations on animal species (birds, alligators, molluscs, fish) and their exposure through their environment ; these discussions have been linked to observations on women exposed to Diethylstilbestrol (DES) and their descendants, leading to the progressive emergence of a new environmental and public health issue.
The WHO definition was established in 2002, updated in 2012 and used by the European Union in 2017:
“An endocrine disruptor is an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub) populations.”
The words in this definition reflect the controversies at stake: the WHO does not refer to substances which “interact with” but which “alter” the endocrine system, nor does it refer to “effects” but to “adverse effects”. There are indeed numerous substances, physical agents or even psychological stress factors which interact with the endocrine system without necessarily causing any adverse effect.
When characterizing an endocrine disruptor, both interaction and toxicity should be demonstrated and toxicity should be proved to derive from the interaction.
Multiple mechanisms of action, requiring multiple studies
The analysis from anses features several mechanisms at play, see also INSERM. Endocrine disruptors can :
- Mimic the action of a natural hormone, thereby causing the response due to this hormone –> this is the mimetic or agonist effect
- Prevent a hormone from binding to its receptor and thus prevent the transmission of the hormonal signal >> this is the blocking or antagonistic effect
- Disrupt the production/degradation or regulation of hormones or their receptors
- Disrupt the transport of a hormone within the organism.
3 factors further accentuate the complexity of the matter :
- Non-monotonic dose-response relationships (a lower dose does not necessarily mean reduced effects, at least within a certain dose range)
- The concept of “windows of exposure” (susceptibility to endocrine disruptors may vary with stages of life)
- Potential “cocktail effects” (one musttake into account the exposure to a mixture of chemicals and understand their interactions).
Last, the fact that the human species is not the only one needing protection.
A multitude of suspected impacts
A series of human health disorders are now suspected to be a consequence of exposure to endocrine disruptors: decrease in sperm quality, increased frequency of abnormalities in organ development or reproductive function, lowering of the age of puberty onset etc. Endocrine disruptors are also suspected to play a part in some hormone-dependant cancers, in some cases of type 2 diabetes, obesity or autism. And questions are being raised about a decline in IQ in the European Union.
EDs are also suspected to play a part in biodiversity decline. Studies on fish intersexuality have shown it is occurring in the environment.
Long lists of suspected substances, yet little « known » substances
The amount of suspected substances is very high, spanning from 10 to a thousand depending on the list – with some ambivalence as some lists of substances which have yet to be investigated are presented as lists of endocrine disruptors.
Those often mentioned are families of substances, such as dioxins, furans, polycyclic aromatic hydrocarbons (PAH), phthalates, bisphenols, parabens, organochlorines, tin and its derivatives – each family encompassing dozens of substances which may or may not share the same properties.
These lists are often limited to manufactured chemical substances. They do not always feature natural hormones which may be synthetised for their use in human or veterinary medicines, and whose waste may non-intentionally impact the environment (vs. the intentional impact of the medicine). These lists do not necessary mention either pollutants or substances which have been banned for a long time but are persistent in our environment.
Alongside these long lists are shorter lists of substances which are investigated in various programs and a low number of known EDs. For instance, around 20 (families of) substances are identified as EDs in REACh, some of these families being very large.
A wide and diverse range of industries concerned
Every industry and business sector is concerned, whether indirectly through plants release, through their finished products or, even if more rarely, through their manufacturing process.
The EU recently published an evaluation (« fitness check ») on how regulations take the issue of EDs into account. More than 30 regulations have been examined, including the following: chemicals, toys, food contact materials, cosmetics, pharmaceutical products (for their impact on the environment), food additives, fertilisers, waste and recycling, plant protection products and biocides, detergents, medical devices, electrical and electronic equipment.
A low level of trust in regulations
The fact that a hefty debate exists around EDs is already a signal. Surveys on the topic of risk perception – such as the IRSN (Institut de Radioprotection et de Sureté Nucléaire) barometer – allow to characterize and detail the issues at stake.
A lack of test methods
Test methods do exist, namely for the assessment of estrogenic and androgenic activity, as well as thyroid and steroidogenesis effects. But it is widely acknowledged that there is a lack of methods in comparison with the amount of endocrine-related effects to be investigated. Hence the call from the European Commission “to progress in the development of internationally agreed test guidelines.”
There is a striking gap between the strong impact suggested by epidemiological research on diabetes, obesity, IQ on the one hand, and the lack of test methods for these issues on the other hand.
Neurodevelopmental issues and metabolic disorders, where research gaps are known to exist, are being addressed in European research programs to better understand the mechanisms at play and develop new methods ( EURION European projects).
Midway between these research programs and already validated methods, some methods do exist, which are technically mature and can go straight away through the pre-validation process. PEPPER’s mission is to accompany the next generation of methods, those which are under development today.
A key regulatory tool : the validated biological assay
In order to use a definition to characterize a substance within a regulatory context, a testing method must be established, which is used to demonstrate the characterization.
For chemicals, we resort to biological assays to demonstrate effects and mechanisms on cells or tissues (in vitro) or on whole organisms (in vivo). Analogies, resemblances and biological modelling, alongside non-validated in vitro and in vivo methods, require a case-by-case approval by the experts. The situation is the same with epidemiology – which is also evidently not applicable to new substances.
Pre-validation is built as an upstream process which will later facilitate and fast-forward validation by international authorities.
The urgency to establish a clear and consistent framework
The debate around endocrine disruptors is characterized by instability and uncertainties, at the crossroads of human health and environment issues, impacts on regulations, the economy and public trust.
Efforts to faster improve risk management exist across all these domains. The European Union has established a framework on the future of the EU’s approach regarding endocrine disruptors and supports research projects. France has launched its second National Strategy for Endocrine Disruptors and aims at leaving uncertainty behind by asking anses to produce a list of EDs.
PEPPER is part of this ecosystem working towards the prompt acknowledgment of characterization methods.
But assays must be validated by an international authority, implying mutual acceptance by its members. Validation requires guarantees on quality and all members to agree on the quality criteria to be satisfied. The principle is that every laboratory working on an assay must obtain the same results. The result obtained with the test method can be used in regulatory dossiers for e.g. REACh, the Water Framework Directive, on the biocides, pesticides, medical devices regulations.
The process undergoes altogether a very technical phase and an approval phase, all led by OECD, EURL ECVAM and ISO.
The length of the process constitutes a weakness. Moving from research to a more operational tool (« translational research »), followed then by reliability assessment, takes time.
PEPPER’s timeline
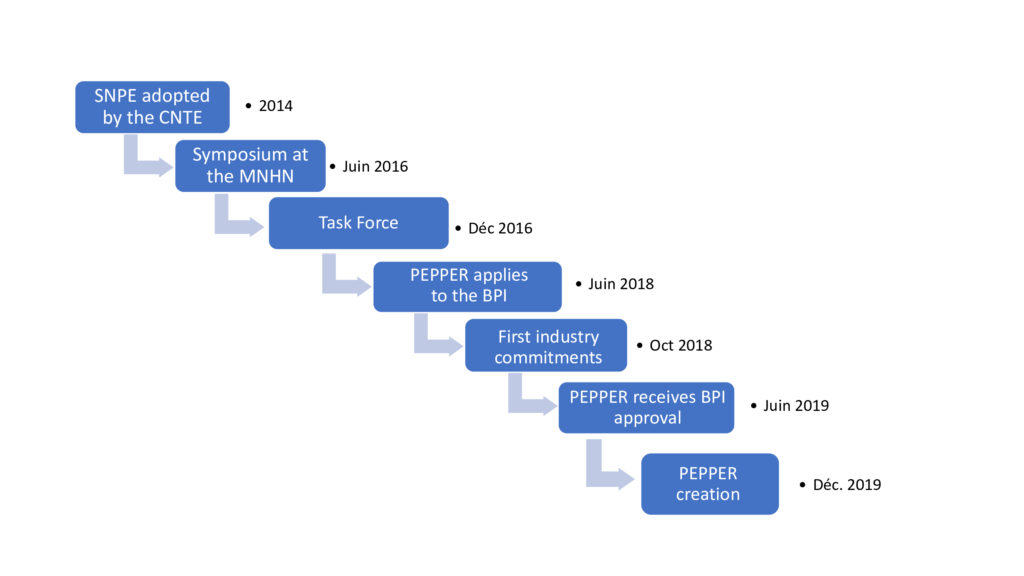
SNPE : National Strategy on EDs.
CNTE : National Council for Ecological Transition.
MNHN : National Museum of Natural History.
